Bioanalytical Testing Services Market Anticipated to Reach $12.24 Billion by 2034, Growing at a CAGR of 9.70%
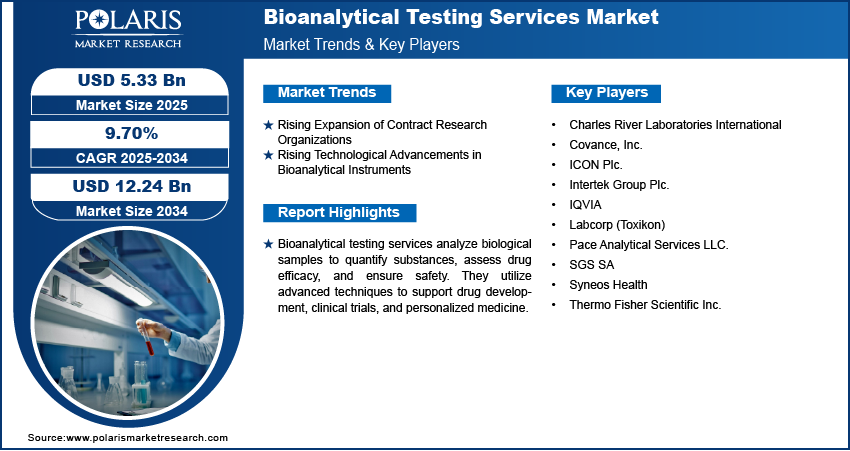
The global Bioanalytical Testing Services Market was valued at USD 4.87 billion in 2024 and is projected to grow at a CAGR of 9.70% from 2025 to 2034.
Key Market Trends & Insights:
- Shift Toward Biologics and Biosimilars:
The rising development of monoclonal antibodies, vaccines, and gene therapies is driving demand for highly sensitive bioanalytical techniques such as ligand-binding assays and LC-MS/MS. - Adoption of Hybrid Analytical Platforms:
Integration of bioanalytical tools like mass spectrometry with ligand-binding assays improves detection capabilities for complex biologics and biomarkers. - Increasing Outsourcing by Pharma Companies:
To reduce operational costs and gain specialized analytical expertise, pharmaceutical firms are increasingly outsourcing bioanalytical testing to CROs. - Regulatory Emphasis on Quality and Compliance:
Enhanced regulatory scrutiny around data integrity, Good Laboratory Practice (GLP), and assay validation is encouraging the use of standardized and advanced testing methodologies. - Growth in Biomarker Discovery and Companion Diagnostics:
Personalized medicine initiatives are fueling the need for biomarker validation services to support targeted drug development and patient stratification.
Market Size & Forecast
Market size value in 2025 USD – 5.33 billion
Revenue forecast in 2034 USD – 12.24 billion
CAGR – 9.70% from 2025 – 2034
Request for Free Sample:
Market Overview:
The bioanalytical testing services market plays a vital role in the development and approval of pharmaceuticals, biologics, and biosimilars. These services include the quantitative and qualitative analysis of drugs, metabolites, and biomarkers in biological samples, ensuring safety, efficacy, and compliance with regulatory guidelines. The market has seen sustained growth due to increasing R&D investments, a rise in biologic drug development, and growing reliance on outsourcing by pharmaceutical and biotechnology companies.
Market Growth Drivers:
- Rising R&D Expenditure:
Pharmaceutical and biotech companies are increasing investment in clinical trials and drug development, boosting demand for bioanalytical services. - Expansion of Clinical Trial Activity:
A growing number of clinical trials, particularly for oncology, autoimmune, and rare diseases, is creating demand for robust testing services across all phases. - Technological Advancements:
Innovations in LC-MS/MS, immunoassays, and automation are enhancing testing accuracy, sensitivity, and throughput. - Supportive Regulatory Frameworks:
Clear guidance from global regulatory bodies has streamlined assay development, validation, and submission processes, making services more accessible. - Increased Focus on Drug Safety and Efficacy:
Rising pressure to demonstrate safety and efficacy before market entry has made bioanalytical testing a critical part of the drug approval process.
Market Challenges:
- High Operational and Setup Costs:
Establishing GLP-compliant laboratories and acquiring advanced instrumentation requires substantial investment, limiting market entry for new players. - Skilled Workforce Shortage:
The demand for experienced Bioanalytical Testing Services scientists outpaces supply, creating talent gaps that can delay projects and reduce service quality. - Data Management and Compliance Burdens:
Managing large volumes of sensitive data and ensuring 21 CFR Part 11 compliance is resource-intensive and error-prone without robust systems. - Complexity of Biologic Drug Testing:
Biologics require highly customized and sensitive analytical methods, increasing development timelines and regulatory scrutiny. - Global Variability in Regulatory Requirements:
Differences in regulatory expectations across regions can complicate multi-site studies and increase the cost and complexity of compliance.