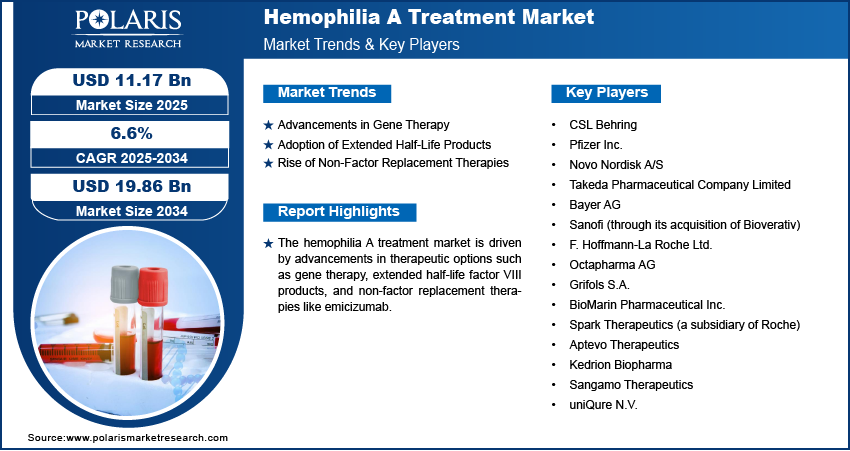
Hemophilia A Treatment Market Projected to Reach USD 19.86 Billion by 2034, Growing at a CAGR of 6.6%
The Hemophilia A treatment market is undergoing a major transformation, fueled by the growing adoption of long-acting therapies, significant advances in gene therapy, and increased focus on personalized care. Hemophilia A is a genetic bleeding disorder caused by a deficiency in clotting factor VIII, leading to prolonged bleeding episodes. The disorder primarily affects males and is one of the most common forms of hemophilia worldwide.
Hemophilia A Treatment Market Trends & Insights
-
Gene Therapy Advancements: Recent approvals of Roctavian (valoctocogene roxaparvovec) have positioned gene therapy as a potential one-time treatment option. These therapies are expected to revolutionize the market by providing sustained production of clotting factors.
-
Long-acting and Subcutaneous Therapies: Innovations such as Altuviiio and concizumab allow less frequent dosing and more convenient administration, enhancing patient adherence.
-
Non-factor Therapies: Bispecific antibodies like emicizumab (Hemlibra), which bypass the need for factor VIII, are gaining popularity due to their efficacy and reduced risk of inhibitor development.
-
Shift Toward Personalized Medicine: Pharmacogenomics and advanced diagnostics are enabling tailored treatment plans based on individual patient profiles and bleeding patterns.
Market Size & Forecast
Market size value in 2025 USD – 11.17 billion
Revenue forecast in 2034 USD – 19.86 billion
CAGR – 6.6% from 2025 – 2034
Request for Free Sample: https://www.polarismarketresearch.com/industry-analysis/hemophilia-a-treatment-market/request-for-sample
Market Overview
The treatment landscape for Hemophilia A includes replacement therapies using recombinant or plasma-derived factor VIII, non-factor therapies such as bispecific antibodies (e.g., emicizumab), and emerging gene therapies aimed at long-term disease correction. Treatment modalities can be classified into on-demand therapies (administered during bleeding episodes) and prophylactic therapies (regular dosing to prevent bleeds). Prophylaxis is becoming the standard of care due to its ability to significantly improve quality of life and reduce long-term joint damage.
Key Market Growth Drivers
-
Rising Prevalence and Awareness: Increased awareness and better screening have led to early diagnosis and treatment, especially in developing regions.
-
Strong R&D Pipeline: Pharmaceutical companies are heavily investing in clinical trials for novel therapies, including gene therapy and monoclonal antibodies.
-
Favorable Regulatory Environment: Orphan drug designations and fast-track approvals by agencies like the FDA and EMA are accelerating market access.
-
Growing Preference for Prophylaxis: A global shift from on-demand to prophylactic treatment is driving the uptake of high-value therapies.
-
Improved Access in Emerging Markets: Government and NGO initiatives are expanding access to affordable treatment in underserved regions.
Market Challenges
-
High Treatment Costs: Biologics and gene therapies can cost hundreds of thousands of dollars annually, limiting accessibility, particularly in low- and middle-income countries.
-
Inhibitor Development: Some patients develop antibodies against factor VIII, complicating treatment and requiring more expensive or alternative therapies.
-
Limited Healthcare Infrastructure: In many regions, inadequate diagnostic capabilities and treatment facilities restrict market penetration.
-
Safety and Efficacy Concerns: Long-term data for newer therapies, especially gene therapies, are still limited, raising questions about durability and potential immune responses.