Rapid Medical Diagnostic Kits Market Projected to Reach $57.6 Billion by 2034, Expanding at a CAGR of 5.80%
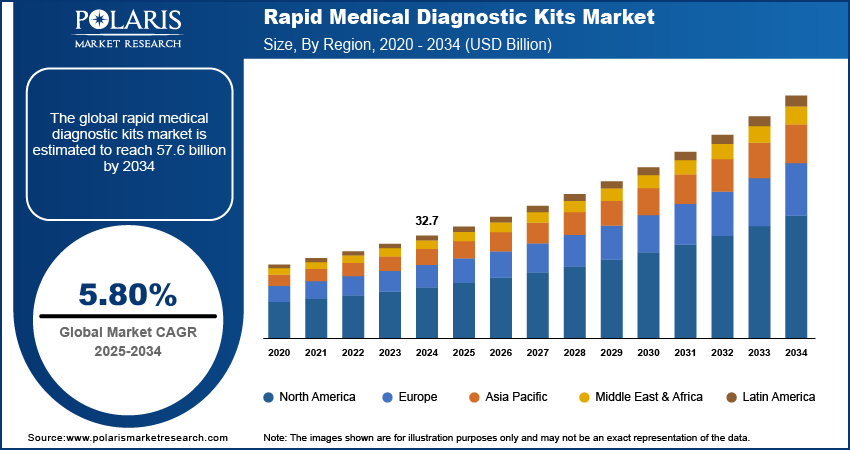
The global rapid medical diagnostic kits market was valued at USD 32.7 billion in 2024 and is expected to grow at a CAGR of 5.80% from 2025 to 2034, fueled by rising demand for point-of-care testing and the need for early and accurate disease detection.
Key Market Trends & Insights:
- Rising Demand for Point-of-Care Testing: Growing preference for quick and accessible diagnostics in clinics, homes, and remote settings.
- Increased Focus on Infectious Disease Detection: Surge in demand due to outbreaks like COVID-19, flu, dengue, and other contagious illnesses.
- Technological Advancements: Integration of AI, biosensors, and microfluidics enhancing accuracy and ease of use in rapid test kits.
- Expansion in Home-Based Healthcare: Consumer shift toward self-diagnosis driving growth in over-the-counter rapid diagnostic products.
- Supportive Government Initiatives: Public health programs and investments in rapid testing infrastructure boosting market adoption.
- Rising Chronic Disease Prevalence: Growing need for fast diagnostics for conditions like diabetes and cardiovascular diseases.
- Emerging Markets Driving Growth: Increasing healthcare access and awareness in Asia-Pacific, Latin America, and Africa fueling demand.
Market Size & Forecast
- Market size value in 2025 – USD 34.6 billion
- Revenue forecast in 2034 – USD 57.6 billion
- CAGR – 5.80%% from 2025 – 2034
Request for Free Sample: https://www.polarismarketresearch.com/industry-analysis/rapid-medical-diagnostic-kits-market/request-for-sample
Key Market Growth Drivers –
- Rising Demand for Point-of-Care Testing (POCT): Increasing need for quick, accurate, and accessible diagnostic results, especially in remote or resource-limited settings, fuels demand for rapid test kits.
- Growing Prevalence of Infectious Diseases: The global rise in infectious diseases such as influenza, COVID-19, malaria, and HIV is significantly boosting the use of rapid diagnostic tools for timely intervention.
- Supportive Government Initiatives: Public health programs and global health organizations are investing heavily in diagnostics to improve disease surveillance, early detection, and treatment outcomes.
- Technological Advancements: Innovations in lateral flow assays, biosensors, and microfluidics are enhancing the sensitivity, portability, and speed of diagnostic kits.
- Increasing Home Healthcare Trend: Growing consumer awareness and preference for at-home testing solutions are expanding the market for user-friendly and non-invasive diagnostic kits.
Market Segmentation:
The rapid medical diagnostic kits market is segmented by product type (lateral flow assays, dipsticks, etc.), technology (immunoassays, molecular diagnostics), application (infectious diseases, glucose monitoring, pregnancy testing), end user (hospitals, home care, labs), and distribution channel (retail, online, direct sales). Regionally, it covers North America, Europe, Asia-Pacific, Latin America, and Middle East & Africa, with Asia-Pacific expected to grow fastest.