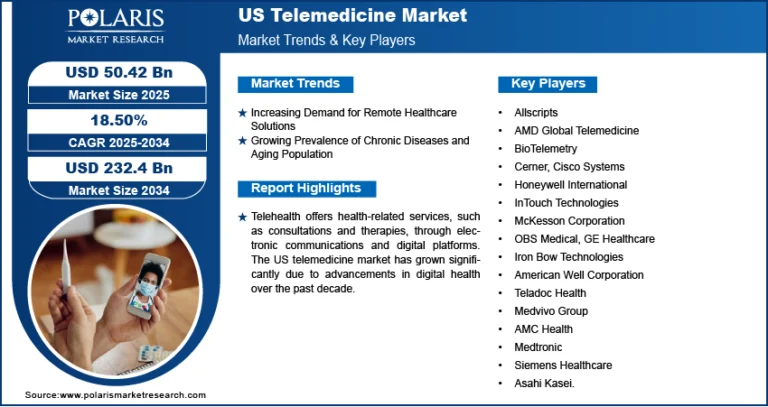
EMEA Point-Of-Care Diagnostics Market Estimated to Reach USD 22.66 Billion by 2034, with a CAGR of 6.1%

Global EMEA Point-Of-Care Diagnostics Market size and share is currently valued at USD 12.56 billion in 2024 and is anticipated to generate an estimated revenue of USD 22.66 billion by 2034, according to the latest study by Polaris Market Research. Besides, the report notes that the market exhibits a robust 6.1% Compound Annual Growth Rate (CAGR) over the forecasted timeframe, 2025 – 2034
Market Definition
The EMEA (Europe, Middle East, and Africa) Point-Of-Care (POC) Diagnostics Market focuses on medical testing conducted at or near the patient site for rapid diagnosis and decision-making. These diagnostics include glucose monitors, rapid antigen tests, pregnancy kits, cardiac markers, and infectious disease detection tools. The market benefits from decentralized healthcare trends, rising chronic disease incidence, and demand for faster clinical workflows. Technological advances in biosensors, microfluidics, and connectivity are enhancing test accuracy and portability. Growing investments in home healthcare, rural health access, and pandemic preparedness are also expanding POC adoption. Regulatory reforms, national health initiatives, and telemedicine integration across the EMEA region support market growth. The shift toward value-based care and personalized medicine continues to drive innovation and scalability in this sector.
Key Report Highlights
- The report highlights the key region that accounts for the highest revenue share in the global EMEA Point-Of-Care Diagnostics market.
- It identifies the leading country within this region that makes a significant contribution to the market’s overall performance.
- The report outlines the dominant segment that holds a major share of the market.
- It also emphasizes the fastest-growing segment projected to gain strong traction during the forecast period.
- Qualitative and quantitative market analysis have been used to provide an in-depth understanding of the market.
Get access to the full report or request a complimentary sample for in-depth analysis:
Market Growth Drivers
The EMEA Point-of-Care Diagnostics Market is growing significantly due to increasing demand for rapid, decentralized healthcare solutions across Europe, the Middle East, and Africa. Rising prevalence of chronic and infectious diseases—such as diabetes, cardiovascular conditions, and respiratory infections—drives the adoption of quick diagnostic tests. Technological advancements in biosensors, microfluidics, and mobile integration are improving the accuracy and accessibility of point-of-care (POC) devices. Growth is supported by government initiatives promoting early diagnosis and primary care access, especially in rural and underserved regions. Additionally, COVID-19 accelerated investment in diagnostic infrastructure and heightened awareness of home-based testing. Expanding public-private partnerships and favorable reimbursement policies are also fueling market expansion. As health systems increasingly emphasize preventative care and real-time monitoring, POC diagnostics will play a crucial role in improving clinical outcomes.
Market Key Players
The competitive landscape features a mix of long-standing companies and emerging contenders. EMEA Point-Of-Care Diagnostics Market Leading players are actively pursuing R&D initiatives and strategic moves to strengthen their market position. Notable participants include
- Abbott
- BD
- BIOMÉRIEUX
- Danaher Corporation
- F. Hoffmann-La Roche Ltd
- Nova Biomedical
- QIAGEN
- Siemens Healthcare Private Limited
- Trividia Health, Inc.
- Werfen