Leukemia Therapeutics Market growing at a CAGR of 7.43% from 2025 to 2033
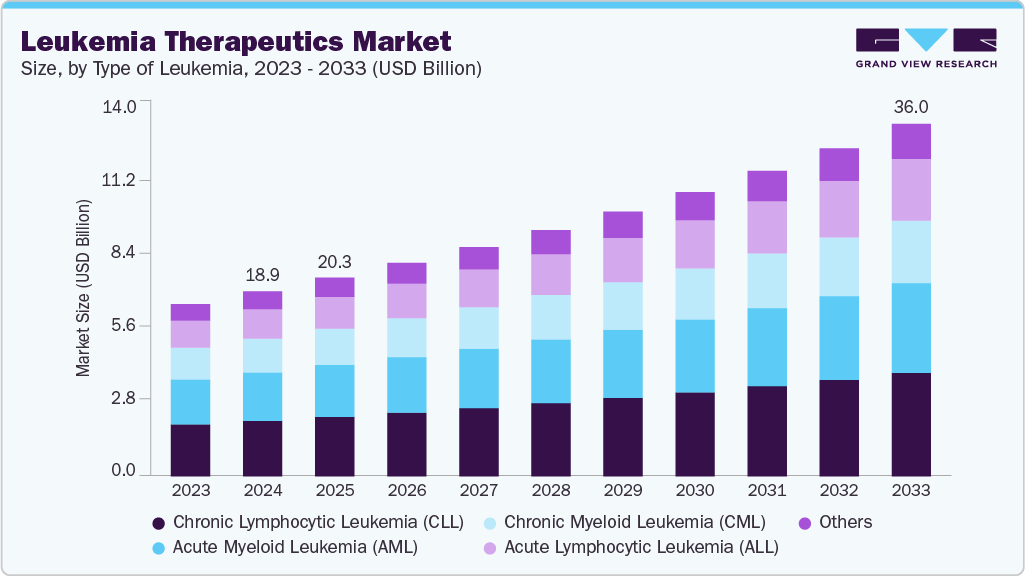
The global leukemia therapeutics market size was estimated at USD 18.90 billion in 2024 and is projected to reach USD 36.02 billion by 2033, growing at a CAGR of 7.43% from 2025 to 2033. The market is expanding due to a rising global prevalence of acute and chronic leukemia across all age groups.
Key Market Trends & Insights
- The North America leukemia therapeutics market held the largest global share of 42.73% in 2024.
- The leukemia therapeutics industry in the U.S. is expected to grow significantly from 2025 to 2033.
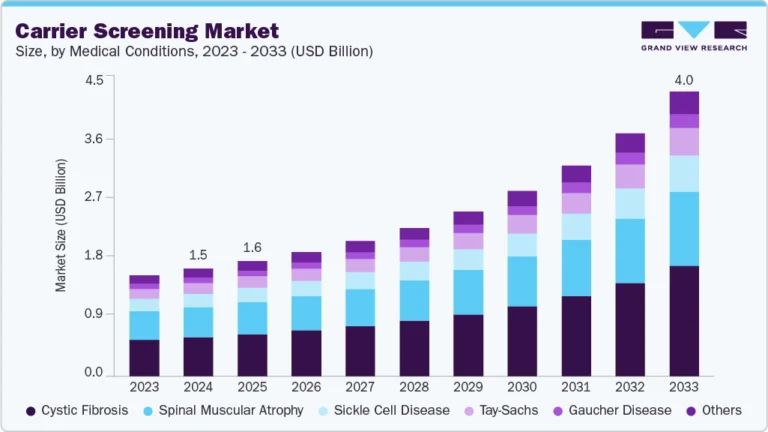
- By type of leukemia, the Chronic Lymphocytic Leukemia (CLL) segment held the highest market share of 30.01% in 2024.
- By treatment type, the targeted therapy segment held the highest market share in 2024.
- By distribution channel, the hospital pharmacy segment held the highest market share in 2024.
Market Size & Forecast
- 2024 Market Size: USD 18.90 Billion
- 2033 Projected Market Size: USD 36.02 Billion
- CAGR (2025-2033): 7.43%
- North America: Largest Market in 2024
- Asia Pacific: Fastest Growing Market
Request Free Sample Report: https://www.grandviewresearch.com/industry-analysis/leukemia-therapeutics-market/request/rs1
The growing adoption of precision oncology is enabling treatments tailored to genetic and molecular profiles, thereby improving patient outcomes. Advancements in diagnostic technologies are enabling earlier detection, which supports higher rates of treatment initiation. Pharmaceutical innovation in targeted therapies and immunotherapies is accelerating clinical adoption and shifting treatment standards. For instance, in April 2025, Frontiers in Medicine published a global burden study which reported that in 2021, there were approximately 461,422.7 leukemia incident cases, projected to reach 509,737.3 by 2031, and around 320,283.6 deaths in 2021 expected to rise to 344,694.3 by 2031, while DALYs declined from 10,982,836.2 to 10,785,356.1 during the same period.
Increasing survival rates are creating a larger population requiring long-term and sequential therapy. Strong R&D momentum supported by active clinical trials is introducing new therapeutic classes with improved efficacy. The commercial availability of combination regimens is further driving prescription volume and increasing therapy value.
The rapid penetration of targeted small-molecule drugs is transforming treatment strategies for AML, CLL, and ALL, delivering high response rates with manageable safety profiles. Continuous progress in kinase inhibitors, BCL-2 inhibitors, and monoclonal antibodies is driving a shift away from traditional chemotherapy. CAR-T therapies are gaining adoption due to their ability to achieve durable remission in patients with relapsed or refractory disease. For instance, in March 2025, Therapeutic Advances in Hematology reported a phase I trial in which 7 of 10 AML patients treated with anti-CLL-1 CAR-T achieved complete remission or CRi. All six who later underwent stem cell transplantation were alive at follow-up. At the same time, another trial showed three complete remissions among six patients receiving anti-CD38 CAR-T, with only grade 1-2 CRS and no ICANS events. Growing access to stem cell transplantation, wider use of oral regimens, and active label expansion strategies are strengthening long-term market growth.
Market Concentration & Characteristics
The leukemia therapeutics market is highly innovation-driven, with continuous progress in targeted therapy, immunotherapy, and genetic-based drug development. Research is shifting from broad cytotoxic agents toward precision oncology and next-generation platforms such as CAR-T, bispecific antibodies, and RNA-based therapeutics. Innovation is also centered on improving the durability of remission, minimizing toxicity, and overcoming resistance in cases of relapse or refractory disease. Strong clinical pipelines from major pharma and biotech firms reflect high investment in novel mechanisms of action. The pace of scientific advancement keeps competitive pressure high and shortens product life cycles.
New entrants face high capital requirements due to costly R&D, complex clinical development, and lengthy approval timelines. Strong IP protection and patent exclusivity held by major players limit competition in key drug classes. Clinical trial design in leukemia is complex due to heterogeneous patient populations and evolving treatment standards. Manufacturing capability for cell and gene therapies represents an additional technical and financial barrier. Market access depends on strong medical, regulatory, and distribution expertise, making entry difficult for non-specialized companies.
Leukemia Therapeutics Market Report Scope
|
Report Attribute |
Details |
|
Market size value in 2025 |
USD 20.30 billion |
|
Revenue forecast in 2033 |
USD 36.02 billion |
|
Growth rate |
CAGR of 7.43% from 2025 to 2033 |
|
Base year for estimation |
2024 |
|
Historical data |
2021 – 2023 |
|
Forecast period |
2025 – 2033 |
|
Quantitative units |
Revenue in USD million/billion and CAGR from 2025 to 2033 |
|
Report coverage |
Revenue forecast, company ranking, competitive landscape, growth factors, trends |
|
Segments covered |
Type of leukemia, treatment type, distribution channel, region |
|
Regional scope |
North America; Europe; Asia Pacific; Latin America; Middle East & Africa |
|
Country scope |
U.S.; Canada; Mexico; UK; Germany; France; Italy; Spain; Denmark; Sweden; Norway; China; Japan; India; Australia; South Korea; Thailand; Brazil; Argentina; South Africa; Saudi Arabia; UAE; Kuwait |
|
Key company profiled |
Pfizer Inc.; Novartis; Lupin Ltd.; Amgen Inc.; AbbVie; Johnson & Johnson Services, Inc.; Bristol-Myers Squibb; F. Hoffmann-La Roche; Takeda Pharmaceutical Co Ltd; Sanofi/ Genzyme Corporation |
|
Customization scope |
Free report customization (equivalent up to 8 analysts working days) with purchase. Addition or alteration to country, regional & segment scope. |
|
Pricing and purchase options |
Avail customized purchase options to meet your exact research needs. Explore purchase options |