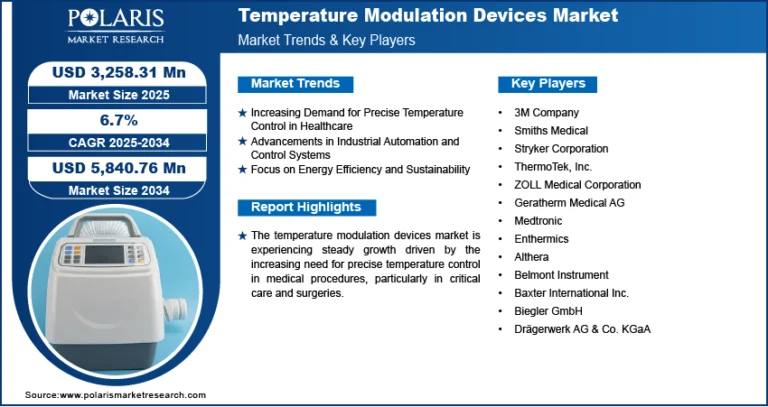
The Mycoplasma Testing Market is expected to attain a value of $4.3 billion by 2034, growing at a CAGR of 13.50%.
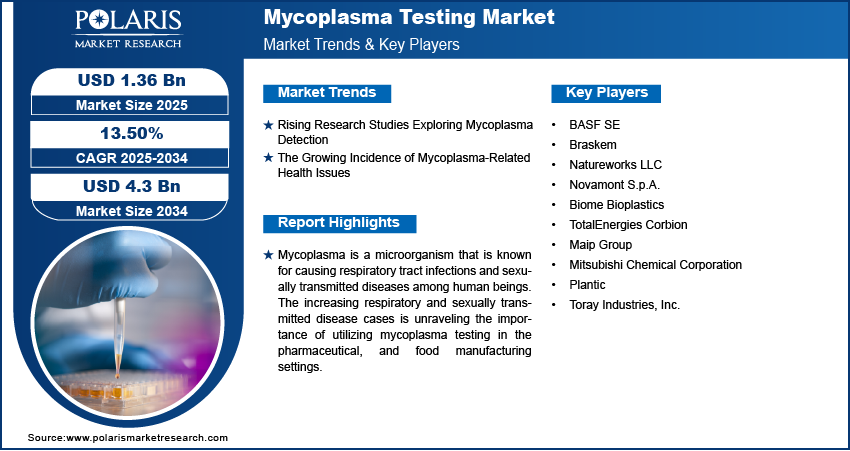
The global mycoplasma testing market was valued at USD 1.2 billion in 2024 and is expected to grow at a CAGR of 13.50% from 2025 to 2034. The market is growing due to heightened biopharmaceutical production and stringent contamination control standards.
Market Trends & Insights:
- Rising Biologics & Biosimilar Production: The expansion of biologics and biosimilar pipelines is increasing the demand for stringent quality control measures, including mycoplasma testing.
- Shift Toward Rapid Detection Methods: PCR-based and nucleic acid amplification technologies are gaining popularity due to their speed and accuracy.
- Increased Regulatory Pressure: Stringent regulatory guidelines from global health agencies are mandating regular mycoplasma screening in manufacturing and R&D environments.
- Automation and High-Throughput Testing: Automation is being integrated into testing protocols to support high-volume workflows and reduce manual errors.
- Adoption in Cell & Gene Therapy: The growing field of cell and gene therapies demands high-quality cell cultures, further boosting market demand for contamination-free systems.
Market Size & Forecast
Market size value in 2025 USD – 1.36 Billion
Revenue forecast in 2034 USD – 4.3 Billion
CAGR – 13.50% from 2025 – 2034
Request for Free Sample:
https://www.polarismarketresearch.com/industry-analysis/mycoplasma-testing-market/request-for-sample
Market Overview:
The Mycoplasma Testing market is gaining momentum as regulatory compliance, biologics development, and cell culture monitoring become increasingly vital in biotechnology and pharmaceutical industries. Mycoplasma contamination poses serious threats to cell lines and bioproduct integrity, prompting organizations to adopt reliable testing methods. The market offers a variety of detection techniques such as PCR, ELISA, and culture-based assays to ensure contamination-free biological products and research environments.
Market Growth Drivers:
- Increased R&D in Life Sciences: The surge in research activities involving cell lines is propelling the need for frequent and reliable mycoplasma detection.
- Stringent Quality Standards: Adherence to good manufacturing practices (GMP) and international testing guidelines is driving adoption across pharmaceutical and biotech firms.
- Expanding Biopharmaceutical Sector: The growth of biopharma manufacturing facilities is creating consistent demand for contamination control measures.
- Awareness and Training Programs: Increased awareness and training on contamination risks and detection methods are supporting broader market penetration.
Market Challenges:
- High Cost of Advanced Testing: Cutting-edge technologies may involve higher implementation and operational costs, limiting adoption among smaller labs.
- Lack of Standardization: Variability in Mycoplasma Testing protocols and result interpretation can lead to inconsistent outcomes across facilities.
- Skilled Workforce Shortage: Performing advanced molecular testing requires technical expertise, which may not be readily available in all regions.
- False Positives/Negatives: Sensitivity issues and sample handling errors can result in misleading outcomes, impacting decision-making and regulatory compliance.