eClinical Solutions Market Projected to Reach USD 36.85 Billion by 2034, Expanding at a CAGR of 13.9%
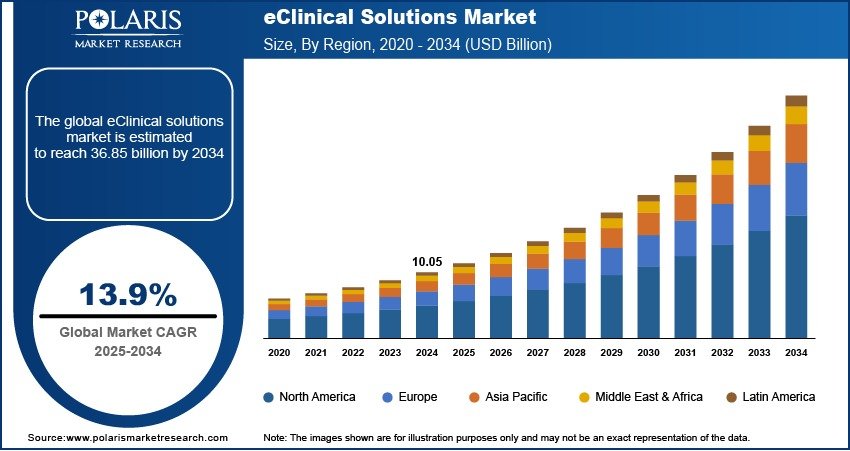
The global eClinical solutions market was valued at USD 10.05 billion in 2024. It is anticipated to expand from USD 11.42 billion in 2025 to USD 36.85 billion by 2034, registering a robust compound annual growth rate (CAGR) of 13.9% over the forecast period from 2025 to 2034.
Key Market Trends & Insights
1. Widespread Adoption of Cloud-Based & Web-Hosted Platforms
- Cloud and web-hosted eClinical solutions are gaining ground due to their scalability, cost-efficiency, remote access, centralized upgrades, and stronger security—fueling faster adoption, particularly in smaller organizations and decentralized trial models.
2. Integration of AI, ML & Big Data Analytics
- AI and ML are being embedded into eClinical tools for tasks like patient recruitment, predictive modeling, automated data extraction (especially from unstructured EHRs), and real-time insights, improving accuracy and efficiency.
3. Shift Toward Decentralized & Virtual Clinical Trials
- Fueled by COVID-19, decentralized (DCTs) and virtual trial models are now mainstream. Telemedicine, remote monitoring, mobile apps, wearables, eConsent, eCOA, and ePRO tools are enhancing patient engagement, comfort, and compliance.
4. Incorporation of Real‑World Data (RWD)
- Increasingly, platforms are pulling in data from wearables, EHRs, and patient-generated records to enrich trials with real-world evidence for deeper insights and personalized outcomes.
Market Size & Forecast
· Market size value in 2025 – USD 11.42 billion
· Revenue forecast in 2034 – USD 36.85 billion
· CAGR – 13.9% from 2025 – 2034
Market Overview
The eClinical solutions market represents a fast-growing segment within the clinical research and pharmaceutical industries, comprising advanced digital tools and platforms that enhance the planning, management, execution, and monitoring of clinical trials. These solutions integrate a range of software applications—including electronic data capture (EDC), clinical trial management systems (CTMS), randomization and trial supply management (RTSM), and electronic patient-reported outcomes (ePRO)—to streamline trial operations and ensure regulatory compliance.
The digitization of clinical research has gained traction in the wake of the COVID-19 pandemic, which highlighted the limitations of traditional paper-based methods. The growing use of remote patient monitoring technologies and digital health platforms has enabled pharmaceutical companies, contract research organizations (CROs), and research institutions to conduct trials more efficiently, even in geographically dispersed locations.
Overall, the eClinical solutions market is being reshaped by innovations in artificial intelligence, cloud computing, and real-time analytics, transforming the way clinical trials are conducted and setting the foundation for more agile, patient-centric research models.
Request for Free Sample: https://www.polarismarketresearch.com/industry-analysis/eclinical-solutions-market/request-for-sample
Key Drivers Accelerating Market Growth
The rapid expansion of the global eClinical solutions market is underpinned by multiple high-impact drivers:
- Rising Volume and Complexity of Clinical Trials: As diseases become more multifactorial, trial designs have grown more complex, involving numerous endpoints and geographic sites. eClinical tools facilitate seamless data collection and remote management across multiple locations.
- Adoption of Decentralized and Hybrid Trial Models: The shift toward patient-centric trials, especially those conducted outside conventional clinical settings, has driven the demand for platforms that support remote patient monitoring and virtual interactions.
- Regulatory Compliance and Data Integrity: With global regulators enforcing strict requirements for traceability, audit trails, and real-time reporting, eClinical platforms offer built-in compliance features that minimize risk and improve documentation standards.
- Growth in Personalized Medicine and Genomic Studies: The increasing use of biomarkers and molecular diagnostics requires advanced data analytics and cross-platform integration—both of which are core capabilities of digital health platforms.
- Cost and Time Efficiency: Traditional clinical trials can take years and millions of dollars to complete. eClinical tools reduce trial duration by up to 30% and lower costs associated with site visits, data entry errors, and manual reconciliation.
Market Challenges
Despite impressive growth potential, the eClinical solutions market is not without challenges:
- Data Privacy and Cybersecurity Risks: Handling sensitive patient data makes eClinical platforms a prime target for cyber threats. Ensuring HIPAA, GDPR, and 21 CFR Part 11 compliance while protecting against breaches is a critical concern.
- Lack of Standardization: The coexistence of multiple vendors and proprietary platforms creates interoperability challenges, limiting seamless data integration across sponsors, CROs, and clinical sites.
- High Implementation and Training Costs: Smaller research organizations and investigator-led trials often lack the budget and IT support needed to deploy sophisticated clinical trial software.
- Regulatory Variability Across Countries: Navigating the differing regulatory landscapes in multi-country trials poses operational delays, particularly in jurisdictions with limited digital health infrastructure.
- User Resistance and Workflow Disruption: Transitioning from manual processes to eClinical platforms often meets resistance from clinical staff unfamiliar with digital interfaces, requiring extensive training and change management.