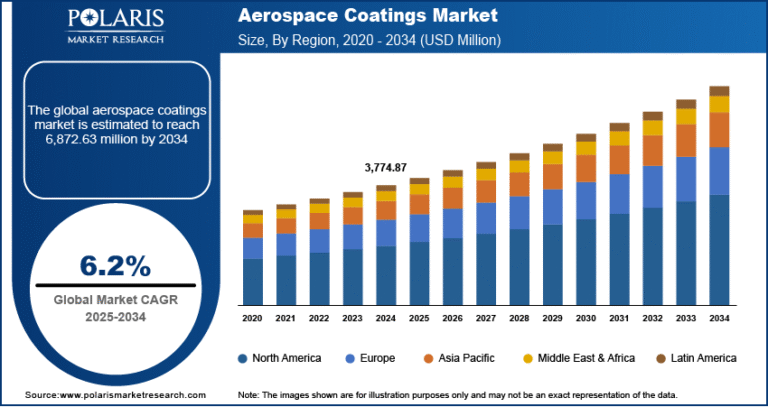
Molecular Diagnostics for Sexually Transmitted Diseases Market to Reach USD 7.85 Billion by 2034, Growing at a CAGR of 7.5%

Molecular Diagnostics For Sexually Transmitted Diseases Market Analysis: Opportunities, Innovations, and Growth Potential Through [2034]
Global Molecular Diagnostics For Sexually Transmitted Diseases Market size and share is currently valued at USD 3.82 billion in 2024 and is anticipated to generate an estimated revenue of USD 7.85 billion by 2034, according to the latest study by Polaris Market Research. Besides, the report notes that the market exhibits a robust 7.5% Compound Annual Growth Rate (CAGR) over the forecasted timeframe, 2025 – 2034
Market Definition:
The molecular diagnostics for sexually transmitted diseases (STDs) market focuses on advanced diagnostic tools that detect the genetic material of pathogens responsible for STDs. These tests offer high sensitivity, rapid results, and early detection of infections such as chlamydia, gonorrhea, HPV, HIV, and syphilis. With increasing global awareness, enhanced healthcare infrastructure, and a growing emphasis on preventive healthcare, molecular diagnostic solutions are becoming a cornerstone in managing and controlling STD transmission effectively.
𝐃𝐨𝐰𝐧𝐥𝐨𝐚𝐝 𝐅𝐫𝐞𝐞 𝐒𝐚𝐦𝐩𝐥𝐞 𝐏𝐃𝐅 𝐂𝐨𝐩𝐲 𝐨𝐟 𝐭𝐡𝐞 𝐑𝐞𝐩𝐨𝐫𝐭:
Key Report Highlights
- The report highlights the key region that accounts for the highest revenue share in the global Molecular Diagnostics For Sexually Transmitted Diseases Market .
- It identifies the leading country within this region that makes a significant contribution to the market’s overall performance.
- The report outlines the dominant segment that holds a major share of the market.
- It also emphasizes the fastest-growing segment projected to gain strong traction during the forecast period.
- Qualitative and quantitative market analysis have been used to provide an in-depth understanding of the market.
Market Overview: Key Figures at a Glance:
|
Report Attributes |
Details |
|
Market Size in 2024 |
USD 3.82 billion |
|
Market Size in 2025 |
USD 4.10 billion |
|
Revenue Forecast by 2034 |
USD 7.85 billion |
|
CAGR |
7.5% from 2025 to 2034 |
𝐂𝐥𝐢𝐜𝐤 𝐡𝐞𝐫𝐞 𝐭𝐨 𝐀𝐜𝐜𝐞𝐬𝐬 𝐭𝐡𝐞 𝐅𝐮𝐥𝐥 𝐑𝐞𝐩𝐨𝐫𝐭:
Market Growth Drivers:
1. Rising Prevalence of Sexually Transmitted Infections Globally:
The increasing incidence of sexually transmitted infections (STIs) is a primary factor fueling the growth of the molecular diagnostics market for STDs. According to global health organizations, millions of new infections occur annually, making STDs a major public health concern. This trend has created a heightened demand for early, accurate, and reliable diagnostic tools. Molecular diagnostics offer quicker and more precise detection compared to conventional methods, thereby supporting timely treatment and preventing complications.
2. Technological Advancements in Molecular Testing:
Significant progress in molecular biology and diagnostic technologies has led to the development of advanced testing methods such as polymerase chain reaction (PCR), nucleic acid amplification tests (NAATs), and point-of-care molecular systems. These technologies provide rapid and highly accurate detection of STD-causing pathogens. The integration of automation, multiplex assays, and user-friendly platforms has enhanced diagnostic efficiency and accessibility, especially in clinical laboratories and decentralized healthcare settings, boosting overall market growth.
3. Growing Public Awareness and Government Initiatives:
Increased awareness about sexual health, driven by educational campaigns and government programs, is encouraging more individuals to undergo routine STD screening. Public health initiatives aimed at reducing the burden of STDs are promoting the use of molecular diagnostics for faster and more reliable detection. Many governments and non-profit organizations are investing in screening and early diagnosis programs, particularly targeting high-risk populations. These proactive efforts are significantly contributing to market expansion.
4. Increasing Adoption in Point-of-Care Testing:
The shift towards point-of-care testing is gaining momentum due to its convenience, speed, and the ability to provide immediate clinical decisions. Molecular diagnostics are increasingly being adapted for portable platforms that can be used in clinics, mobile health units, and community settings. These compact devices are revolutionizing STD testing by providing rapid results in under an hour, facilitating early intervention. This growing trend is especially beneficial in regions with limited access to centralized laboratory facilities.
5. Expansion of Healthcare Infrastructure in Emerging Markets:
Emerging economies are witnessing rapid improvements in healthcare infrastructure, driven by rising healthcare spending and investments in diagnostic technologies. As awareness about STDs increases and diagnostic services become more accessible, molecular diagnostics are gaining traction in these regions. Additionally, the affordability of molecular testing is improving, thanks to increased competition and government subsidies, allowing broader implementation. This expansion in healthcare capabilities is creating substantial growth opportunities for market players worldwide.
Market Key Players
The competitive landscape features a mix of long-standing companies and emerging contenders. Leading players are actively pursuing R&D initiatives and strategic moves to strengthen their market position. Notable participants include:
- Abbott Laboratories
- Becton, Dickinson and Company (BD)
- bioMérieux SA
- DiaSorin S.p. A.
- F. Hoffmann-La Roche AG (Roche Diagnostics)
- Hologic Inc.
- QIAGEN N.V.
- QuidelOrtho Corporation
- Seegene Inc.
- Siemens Healthineers AG
- Thermo Fisher Scientific Inc.




![Gabapentin Market Analysis: Opportunities, Innovations, and Growth Potential Through [2025-2034]](https://beeswire.com/wp-content/uploads/2025/07/polaris-59-768x768.png)