Lewy Body Dementia Treatment Market to Reach USD 13.32 Billion by 2034 | CAGR Projected at 8.1%
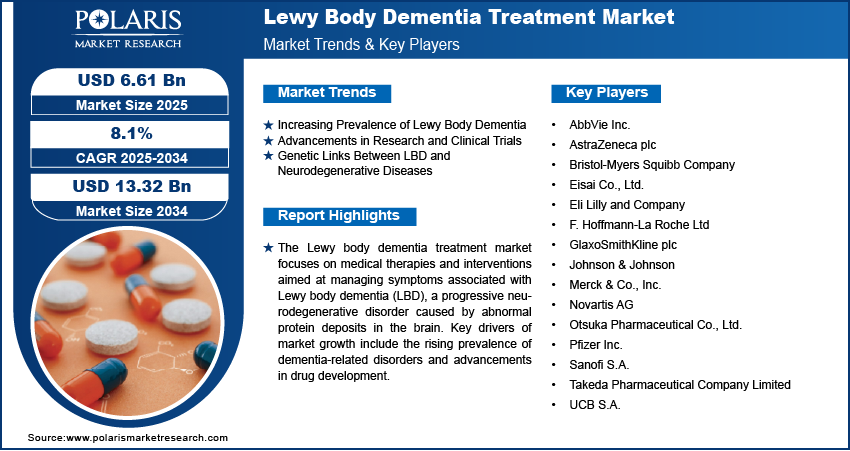
The global Lewy body dementia treatment market was valued at USD 6.13 billion in 2024. It is projected to expand from USD 6.61 billion in 2025 to reach USD 13.32 billion by 2034, reflecting a compound annual growth rate (CAGR) of 8.1% over the forecast period from 2025 to 2034. Key factors driving this growth include the increasing prevalence of dementia-related conditions, advancements in pharmaceutical development, and rising global healthcare expenditures.
Lewy Body Dementia Treatment Market Key Trends & Insights:
- Rapid product innovation: Companies are actively developing new treatments, including disease-modifying therapies such as monoclonal antibodies and alternative delivery systems like transdermal patches, enhancing patient convenience and adherence.
- Emergence of precision medicine & biomarker-driven approaches: The market is witnessing a shift toward personalized treatment plans using genetic profiling and biomarkers, aiming to improve diagnostic accuracy and therapeutic outcomes for individual patients.
- Digital health integration and telemedicine uptake: Technologies such as remote monitoring, telehealth platforms, wearable devices, and virtual reality tools are increasingly being adopted to support continuous care and symptom management, especially post-pandemic.
- Strengthened healthcare infrastructure and awareness efforts: Enhanced focus on public education, increased memory clinics, and specialized dementia care centers are contributing to earlier diagnosis and broader access to treatment.
- Growing therapeutic pipeline with symptomatic and disease-modifying agents: While current therapies largely address symptoms, a strong pipeline of treatments is in development targeting disease mechanisms like alpha-synuclein buildup and neuroinflammation.
Market Size & Forecast:
- Market size value in 2025 – USD 6.61 billion
- Revenue forecast in 2034 – USD 13.32 billion
- CAGR – 8.1% from 2025 – 2034
𝐆𝐞𝐭 𝐄𝐱𝐜𝐥𝐮𝐬𝐢𝐯𝐞 𝐒𝐚𝐦𝐩𝐥𝐞 𝐏𝐚𝐠𝐞𝐬 𝐨𝐟 𝐓𝐡𝐢𝐬 𝐑𝐞𝐩𝐨𝐫𝐭:
Lewy Body Dementia Treatment Market Overview:
The Lewy Body Dementia (LBD) treatment market is evolving steadily, shaped by increasing recognition of LBD as a distinct neurodegenerative disorder. Patient numbers are rising alongside better diagnostic practices, which in turn fuels demand for both pharmacological and non-pharmacological options. Established symptomatic therapies such as cholinesterase inhibitors and motor symptom agents continue to dominate clinical use, while off-label approaches address behavioral and psychiatric manifestations. Emerging focus on tailored care pathways has spotlighted multidisciplinary strategies incorporating neurology, psychiatry, caregiving support, and technology-enabled solutions.
At the same time, the pipeline of novel therapies particularly those targeting alpha-synuclein, neuroinflammation, and neuroprotective mechanisms is drawing attention and investment, albeit still in early development. Regulatory frameworks are adapting to the complexities of LBD trials, and stakeholder collaboration among academia, biotech, and patient advocacy groups is strengthening. Key challenges persist, however, including misdiagnosis, fragmented reimbursement policies, and the absence of disease-modifying treatments. Looking forward, the market’s trajectory is expected to be shaped by advancements in biomarker-driven research, innovation in trial design, and integrated care delivery models.