Rare Disease Genetic Testing Market Projected to Reach USD 3,790.38 Million By 2034, Growing at a CAGR of 13.2%
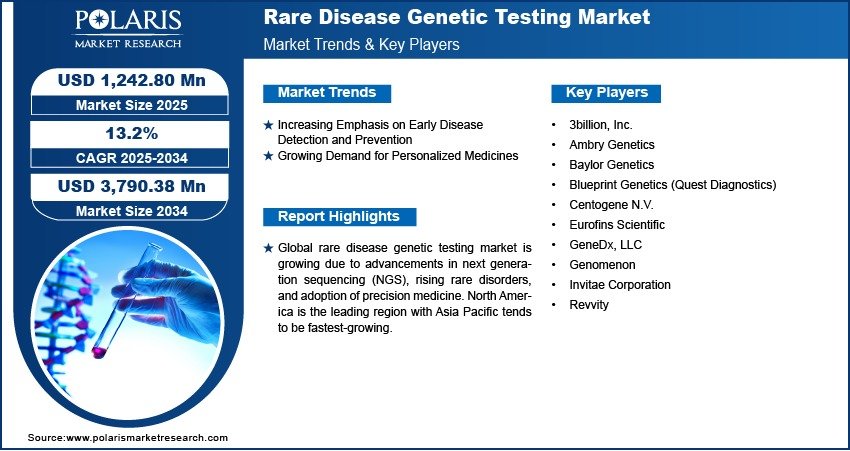
The global Rare Disease Genetic Testing market size was valued at USD 1,106.88 million in 2024 and is expected to grow from USD 1,242.80 million in 2025 to USD 3,790.38 million by 2034 , exhibiting a Compound Annual Growth Rate (CAGR) of 13.2% during the forecast period from 2025 to 2034 .
- Rising Prevalence of Rare Genetic Disorders: The increasing incidence of rare diseases—most of which have a genetic origin—is driving demand for early diagnosis through advanced genetic testing methods.
- Advancements in Genomic Technologies: Innovations in next-generation sequencing, (NGS), whole genome sequencing (WGS), and whole exome sequencing (WES) are enabling faster, more accurate, and cost-effective diagnosis of rare diseases.
- Growing Awareness About Early Diagnosis: Governments, healthcare providers, and patient advocacy groups are emphasizing the importance of early detection and treatment of rare diseases, leading to increased adoption of genetic testing across both developed and emerging markets.
- Expansion of Newborn Screening Programs: Many countries are integrating genetic screening into national newborn screening programs to detect rare disorders at an early stage, thereby improving treatment outcomes and reducing long-term healthcare costs.
- Integration with AI and Big Data Analytics: AI-powered diagnostics, machine learning algorithms, and bioinformatics tools are enhancing data interpretation and diagnostic accuracy in rare disease testing, supporting market expansion.
Market Size & Forecast
- Market Size in 2024 – USD 1,106.88 million
- Market Size in 2025 – USD 1,242.80 million
- Projected Market Size by 2034 – USD 3,790.38 million
- CAGR (2025–2034) – 13.2%
Rare disease genetic testing involves the use of molecular diagnostic techniques to identify genetic mutations responsible for rare inherited conditions. These tests play a crucial role in diagnosing rare diseases that affect fewer than 200,000 people in the U.S., or less than 1 in 2,000 individuals in Europe. Accurate diagnosis enables tailored treatment strategies, supports clinical trial enrollment, and facilitates informed decision-making for patients and families.
The market is experiencing strong growth driven by advancements in genomic technologies, rising government funding for rare disease research, and growing investments by biopharmaceutical companies in orphan drugs and gene therapies. Additionally, the integration of digital health platforms, telemedicine-based genetic counseling, and personalized medicine initiatives is further fueling adoption globally.
Strategic innovation continues to shape the future of the rare disease genetic testing industry. Companies are investing in multi-gene panels, liquid biopsy-based testing, and cloud-based genomic data management systems to improve accessibility and turnaround time. Strategic partnerships between diagnostic laboratories, biotech firms, and regulatory agencies are also accelerating the development of targeted therapies and companion diagnostics. As precision medicine gains momentum, the rare disease genetic testing market is poised for significant expansion over the next decade.